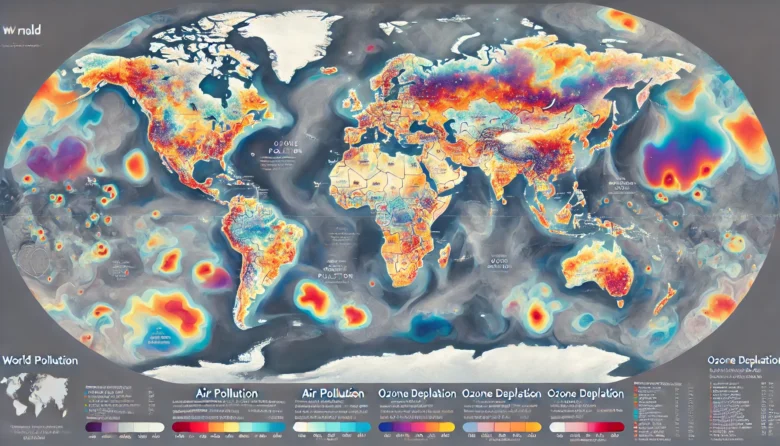
Imagine a world without the protective shield of the ozone layer, where stepping outside could mean exposing yourself to the sun’s harmful ultraviolet (UV) radiation. Or consider the impact of polluted air, thick with smog, making breathing difficult. These scenarios might seem like dystopian fantasies, but they highlight the critical role of the atmosphere’s chemistry in protecting life on Earth. In this blog, we’ll explore the atmosphere’s chemistry, focusing on the ozone layer and pollution, and explore how these factors are interconnected in maintaining the fragile equilibrium that supports life on Earth.
The Ozone Layer: Earth’s Natural Sunscreen
The ozone layer is often referred to as Earth’s natural sunscreen and for a good reason. Located in the stratosphere, about 15 to 35 kilometres above the Earth’s surface, the ozone layer contains a high concentration of ozone (O₃) molecules. This layer plays a crucial role in absorbing most of the sun’s harmful UV radiation, particularly UV-B and UV-C rays, which can result in skin cancer, cataracts, and other serious health problems in humans while also harming ecosystems.
Ozone itself is a molecule made up of three oxygen atoms. It forms in the stratosphere when UV light splits oxygen molecules (O₂) into individual oxygen atoms, which then combine with other oxygen molecules to create ozone. This process is a natural and continuous cycle that maintains the ozone balance in the atmosphere.

However, human activities have disrupted this balance. The most significant threat to the ozone layer came from chlorofluorocarbons (CFCs), substances that were commonly used in refrigeration, air conditioning, and aerosol products. When CFCs reach the stratosphere, UV radiation breaks them down, releasing chlorine atoms that react with ozone, breaking them down into oxygen molecules and depleting the ozone layer.
A dramatic example of this was the discovery of the ozone hole over Antarctica in the 1980s. This “hole” was not an actual gap but a region where the ozone concentration was significantly reduced, allowing more UV radiation to reach the Earth’s surface. The global community responded to this crisis with the Montreal Protocol, an international treaty established in 1987 to phase out the production and use of substances that deplete the ozone layer. These efforts have led to a gradual recovery of the ozone layer. However, it will take several more decades to return to its pre-1980 levels.
Air Pollution: The Dark Side of Industrialization
While the ozone layer sits high above us, protecting life on Earth, the air we breathe at ground level is becoming increasingly polluted. Air pollution is a complex blend of gases, particulates, and biological molecules that can harm human health and the environment. Major sources of air pollution include industrial processes, vehicle emissions, agricultural practices, and natural events like wildfires and volcanic eruptions.
One of the most well-known pollutants is particulate matter (PM), composed of tiny particles or droplets in the air. PM is categorized by size, with PM10 referring to particles with 10 micrometres or fewer diameters and PM2.5 for those with 2.5 micrometres or less. These particles are small enough to be inhaled into the lungs, where they can trigger respiratory and cardiovascular issues, especially in vulnerable groups like children and the elderly.
Another major pollutant is nitrogen dioxide (NO₂), a gas generated by the combustion of fossil fuels, particularly in vehicles and power plants. NO₂ can irritate the lungs, reduce immunity to lung infections, and contribute to the formation of ground-level ozone, a key component of smog.
This term originally referred to smog as a mix of smoke and fog, but today, it often describes photochemical smog, which forms when sunlight reacts with pollutants like nitrogen oxides (NOₓ) and volatile organic compounds (VOCs). This type of smog is particularly common in urban areas with heavy traffic. It can cause a range of health problems, including asthma and other respiratory conditions.
A poignant case study is the Great Smog of London in 1952. This severe air pollution event resulted in thousands of deaths. It led to significant changes in environmental regulations in the UK. Recently, cities like Delhi and Beijing have experienced severe air pollution crises, where smog has become a daily part of life. This has prompted governments to implement emergency measures like restricting vehicle use and shutting factories.
The Chemistry of Air Pollution: How Pollutants Interact
Air pollution is not just about the presence of harmful substances in the air; it’s also about the chemical reactions these substances undergo in the atmosphere. For instance, ground-level ozone, harmful to human health, is not directly emitted but forms through a reaction between nitrogen oxides (NOₓ) and VOCs in sunlight. This reaction is a classic example of photochemical smog, where pollutants undergo chemical transformations driven by sunlight.
Another critical reaction involves sulfur dioxide (SO₂), a gas released by burning fossil fuels containing sulfur, such as coal and oil. When SO₂ is emitted into the atmosphere, it can combine with water vapour to form sulfuric acid (H₂SO₄), resulting in acid rain. Acid rain can have severe consequences for ecosystems, damaging forests, soils, and aquatic environments, as well as eroding buildings and monuments.
Moreover, the interaction between different pollutants can exacerbate their effects. For example, the combination of NO₂ and particulate matter can lead to the formation of secondary pollutants like ammonium nitrate, which contributes to PM2.5 levels and poses significant health risks.
Climate Change and Atmospheric Chemistry
The chemistry of the atmosphere is also deeply intertwined with climate change. Greenhouse gases (GHGs), such as carbon dioxide (CO₂), methane (CH₄), and nitrous oxide (N₂O), trap heat within the Earth’s atmosphere, contributing to global warming. Burning fossil fuels, deforestation, and industrial processes have dramatically increased the concentration of these gases, leading to rising global temperatures and more extreme weather patterns.
In addition to their warming effect, some GHGs, like methane, can also contribute to the formation of ground-level ozone. Methane is a powerful GHG that can react with other atmospheric compounds to create ozone, which, as mentioned earlier, is a harmful pollutant at ground level.
The interplay between air pollution and climate change is complex and multifaceted. For instance, black carbon (a component of PM2.5) not only affects human health but also intensifies climate change by absorbing sunlight and warming the atmosphere. Furthermore, reducing air pollution, such as transitioning to cleaner energy sources, can also help mitigate climate change by reducing GHG emissions.
Case Study: The Clean Air Act and Its Impact
The Clean Air Act in the United States is one of the most significant legislative efforts to combat air pollution. Enacted in 1963 and amended several times, this law has played a crucial role in reducing air pollution and improving public health. The Clean Air Act set national air quality standards, establishing limits on emissions from industries and vehicles. It authorized the Environmental Protection Agency (EPA) to enforce these rules.
The impact of the Clean Air Act has been profound. Since it was enacted, emissions of key pollutants like sulfur dioxide, nitrogen oxides, and particulate matter have significantly declined, resulting in cleaner air and improved public health. The success of this legislation has also inspired similar efforts in other countries, highlighting the importance of strong regulatory frameworks in addressing environmental challenges.
Conclusion
The chemistry of the atmosphere, encompassing the ozone layer and pollution, is a critical component of our planet’s health and well-being. Understanding the delicate balance of atmospheric chemistry allows us to appreciate the importance of protecting the ozone layer and reducing air pollution. As individuals and as a society, we must continue to support and advocate for policies and practices that safeguard our atmosphere, ensuring a healthier future for future generations.
Author’s Note
As someone deeply passionate about environmental science, understanding the chemistry of our atmosphere is key to tackling the challenges of ozone depletion and air pollution. By learning more about these issues, we can all help protect our planet and our health.
G.C., Ecosociosphere contributor.