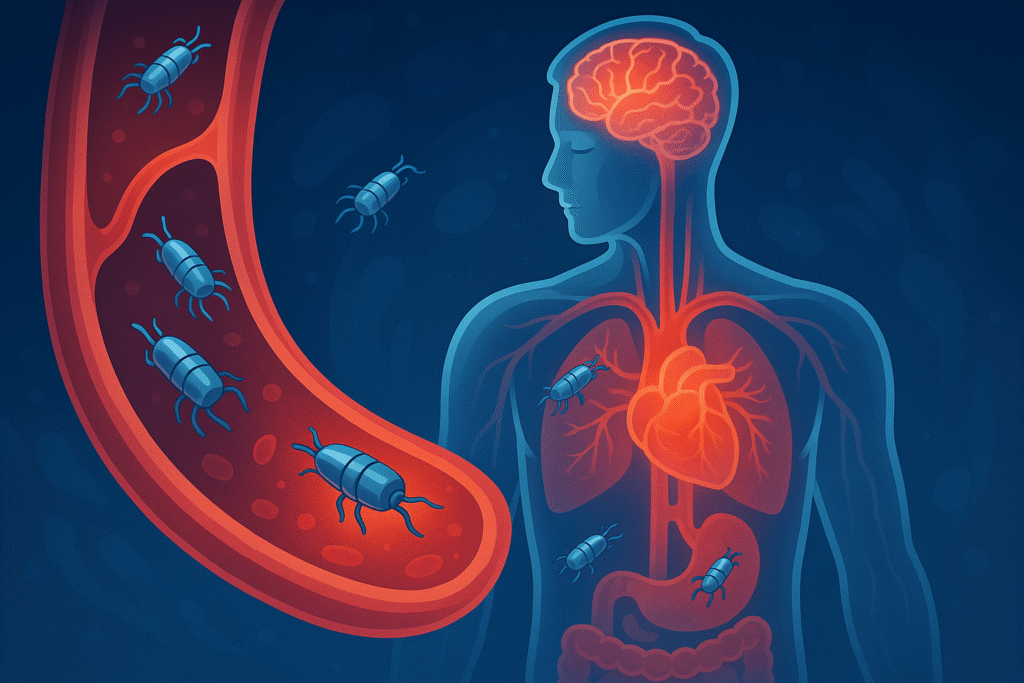
Here’s a fun fact: a robot smaller than a grain of sand may soon swim through your blood — and deliver medicine directly to the right part of you.
This is the story behind Tiny Robots in Blood: What Science Is Doing to Heal Us — a future that seems pulled from science fiction, but is beginning to unfold in labs around the world. Imagine doctors releasing millions of micro-scale couriers into the bloodstream — not to wander aimlessly, but to navigate through arteries and veins, find a clot or a tumour, and deliver drugs exactly where they are needed. No more “spray and pray,” no more heavy doses coursing through the entire body, hitting healthy parts as collateral damage. Instead: precision, minimal side-effects, maximum impact.
Why we need tiny robots
Traditional drug delivery is blunt. When you take a pill or get an injection, the medicine travels through your entire circulatory system. To reach, say, a blood clot in your brain or a tumour deep inside your lungs, you often need a high dose so that a sufficient amount reaches the target — and that often means your healthy organs get exposed too. The result: side-effects, toxicity, sometimes even the end of promising therapies.
That’s why scientists have long searched for a better way — a method that delivers drugs locally, conserving most of the body from harm. Over the years, this search gave rise to nanoparticles and nanomedicine. But even then, many challenges remained: getting the particles to escape blood vessels, accumulate where needed, cross biological barriers, and release the drug in a controlled way.
Enter: microrobots and nanorobots. Robots so small that they can flow through blood vessels, move actively, overcome the rush of blood flow, and steer themselves to diseased sites.
What’s new: “swimming” microrobots that dissolve
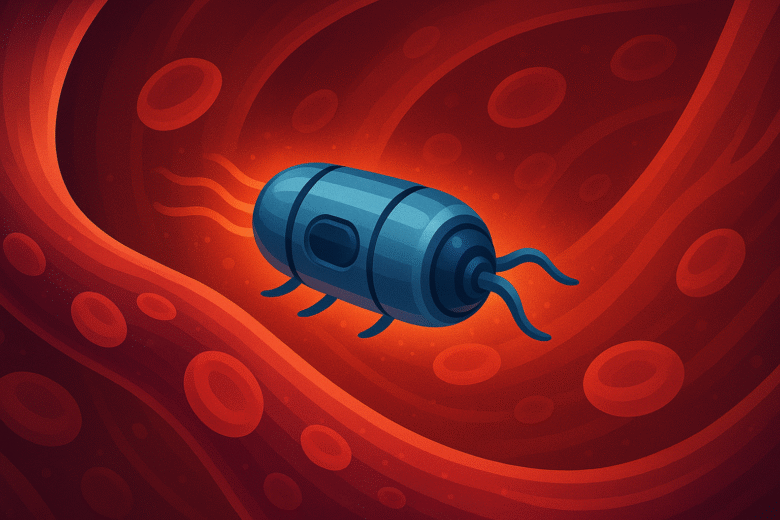
In November 2025, a breakthrough captured headlines: researchers at ETH Zurich unveiled microrobots that can swim through blood, steer under magnetic control, deliver drugs precisely, and then dissolve — leaving nothing behind.
These microrobots are roughly the size of a grain of sand. They’re made from a soluble gel capsule containing the drug payload together with iron-oxide nanoparticles — making them magnetic and steerable via external magnetic fields. To track their journey, they incorporate contrast agents detectable by X-ray imaging.
In lab tests and animal models (with blood vessels comparable to humans), the results are impressive. The microrobots were guided through complex vascular networks — crawling along vessel walls, swimming even against blood flow — with over 95% success at hitting the desired target. Once in place, a controlled trigger (often heat from a magnetic field) causes the capsule to dissolve, releasing its drug payload exactly where needed.
This is not a toy. This is medicine reimagined.
More than one approach — microrobots come in many styles
The ETH Zurich microrobot is a recent — and very promising — star. But it is not the only approach scientists are exploring to bring “robots in blood” to reality.
Biohybrid microrobots: Some designs combine living cells (like bacteria, sperm, or red-blood-cell “ghosts”) with synthetic carriers. These biohybrid swimmers use the natural motility or biological behaviour of cells to navigate complex bodily terrain. Sometimes they come with magnetic or acoustic steering as well.
Surface-active “squirmer” robots: Simulations show that tiny robots which actively rearrange surrounding blood particles to swim — rather than being inert drift-devices — move more efficiently through blood than passive ones. This matters because blood is a dense, complex fluid.
Nanorobots for tumour therapy and chronic disease: Recent reviews suggest that nano/microrobots could redefine how we approach tumour therapy, chronic diseases, and even continuous treatments — offering a future where drugs aren’t just thrown into the bloodstream regularly, but delivered exactly where and when needed.
What could this mean for patients — and healthcare?
The potential is huge.
Consider stroke: right now, clot-busting drugs carry serious risks because they circulate everywhere. A microrobot could travel like a precision-guided missile, deliver the clot-dissolving drug right at the blockage, dissolve, and disappear — minimising collateral risk.
Think about cancer: chemotherapy works because it attacks rapidly dividing cells — but healthy cells often catch the blow too, causing the toxic side-effects people dread. Microrobots could take chemotherapy directly into a tumour’s core, shorten treatment duration, reduce intensity, and spare healthy organs.
Even infections — hard-to-reach ones, or infections deep inside tissues — could one day be treated with robots delivering antibiotics locally, avoiding the systemic overload that antibiotics often bring.
And beyond that, imagine chronic illnesses like kidney disease, vascular diseases, or neurological conditions receiving tailor-made, micro-precision therapy.
But real life is messy: the challenges — and the scepticism
Before we surrender to hope, we need to be honest. Science here is still in early stages. Most of these microrobots have been tested in vessel models or animals — not yet humans.
Blood is more than a fluid: it’s a living, reactive environment full of immune cells, proteins, changing flows. Moving a robot through it — and reliably controlling it — is vastly more complex than guiding a remote-controlled boat. That’s why designs must balance size, safety, control, visibility, and biodegradability.
Then there is the matter of scaling: manufacturing millions of tiny robots that are safe, reliable, and consistent — and gaining regulatory approval for human use — is a huge step. There are ethical, safety, long-term effects to study. Some critics even question whether we are ready to let “machines” roam our insides.
Yet despite all this, the pace of progress is ferocious. What seemed like wild science fiction under the microscope a few years ago is now being tested in animals, and being seriously discussed for hospitals.
Conclusion
Tiny robots swimming in our blood — not to spy, not to harm, but to heal — may sound futuristic. And in many ways, it is. But it also feels like one of those turning points in science: where biology, engineering, and medicine intersect in a deeply human way. Precision healing, lower side-effects, targeted therapy — this could change the way we think about illness and treatment.
Someday soon, a stroke patient could receive a dose aimed only at a struggling clot. A cancer patient might get chemotherapy targeted at the tumour, while their healthy organs rest easy. A chronic patient might avoid repeated heavy dosing, replaced by smart, minimal-invasion interventions.
Of course, we have hurdles — biology is messy, regulation is slow, and fear of new technology is real. But when I think of the promise in these tiny swimmers, I also think of possibility: a world where disease doesn’t always mean collateral damage; where medicine isn’t shotgun-style, but sniper-sharp.
Let’s keep watching, keep demanding safety and ethics, but also allow ourselves to imagine this new landscape of healing.
Author’s Note
I write because I’m fascinated by how human ingenuity can reshape suffering — sometimes with a pill, sometimes with something far stranger. The idea of microrobots swimming through blood unsettles me a little. It feels like allowing machines into our most private places: our veins, our organs, our very lifeblood. And yet it also feels like hope — hope for healing that understands your body, for medicine that treads lightly.
G.C., Ecosociosphere contributor.
References and Further Reading
- Microrobots deliver drugs directly to bloodstream — Science news
- ETH Zurich breakthrough in microrobot drug delivery
- Microrobots swim through blood vessels — Anadolu Agency / international coverage
- Review: Micro/Nanorobots for Biomedical Applications
- Biohybrid microrobots: merging biology and machines
- Surface-active microrobots for efficient blood propulsion