Did you know that water can boil and freeze simultaneously? This mind-bending phenomenon occurs under specific conditions known as the triple point. While it sounds like something out of science fiction, this unique behaviour has real-world implications for our understanding of matter and thermodynamics. The triple point isn’t just a quirky fact; it’s a fundamental concept in physics and chemistry, providing insights into the states of matter and even forming the basis for certain temperature standards.
Understanding the Triple Point
What is the Triple Point?
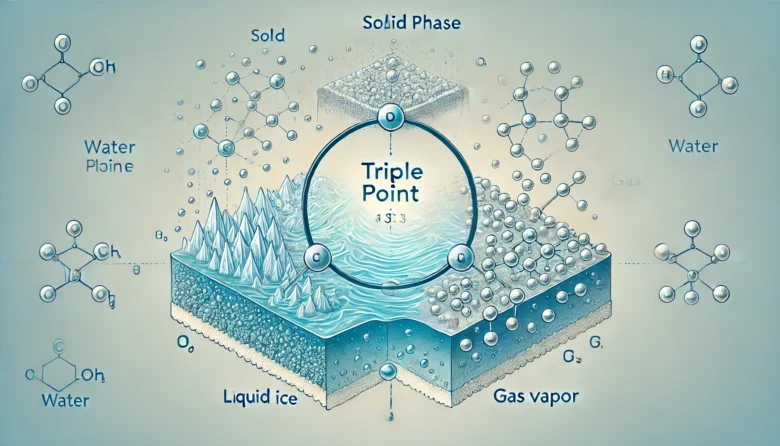
The triple point of a substance is the specific combination of temperature and pressure at which all three states of matter—solid, liquid, and gas—coexist in thermodynamic equilibrium. For water, this occurs at 0.01°C (273.16 K) and a pressure of 611.657 pascals. At this delicate balance, water molecules are transitioning seamlessly between freezing, boiling, and condensation, creating a mesmerizing interplay of phases.
How Does It Work?
To understand the triple point, imagine a sealed container filled with water and its vapour. Adjusting the pressure and temperature precisely to the triple point conditions will cause ice to form, water to boil, and vapour to condense simultaneously. This happens because the energy required for phase changes aligns perfectly at this point.

A Real-World Demonstration
One of the most captivating demonstrations of the triple point involves a vacuum chamber. Scientists reduce the pressure inside the chamber to the triple point of water while maintaining its temperature. As the pressure drops, the water begins to boil. At the same time, due to the low temperature, ice starts forming. This dual behaviour provides a stunning visual of the triple point in action.
Significance in Science
The triple point of water isn’t just a laboratory curiosity; it’s pivotal for several scientific and practical applications:
- Defining Temperature Scales: The triple point of water is used to define the Kelvin scale, ensuring universal standardization in thermometry.
- Studying Phase Transitions: Understanding the triple point helps scientists explore the behaviour of substances under extreme conditions, such as in the interiors of planets or during cryogenic processes.
- Industrial Applications: Industries use the principles of phase transitions for processes like freeze-drying and vacuum distillation.
Challenges and Mysteries
While the triple point is well-documented for water and other substances, it raises intriguing questions about the behaviour of matter in extreme conditions. For instance:
- What happens at the triple point for complex substances like alloys or biological materials?
- How do impurities affect the triple point? These unanswered questions push the boundaries of material science and thermodynamics, inspiring further research.
Conclusion: A Window into Nature’s Wonders
The triple point of water is a fascinating reminder of the complexity and elegance of nature. It bridges the gap between science and wonder, showing us how seemingly mundane substances can exhibit extraordinary behaviours under the right circumstances. As we delve deeper into the mysteries of matter, phenomena like the triple point continue to enrich our understanding of the universe. So, the next time you boil water for tea, take a moment to marvel at the intricate dance of phases beneath the surface.
Author’s Note
I’ve always been fascinated by the hidden wonders of everyday phenomena, and the triple point of water is a perfect example. It’s a small glimpse into the intricate rules that govern our universe, and exploring it feels like unlocking a secret. I hope this blog sparks your curiosity and appreciation for the marvels of science.
G.C., Ecosociosphere contributor.
Further Reading